On Friday, September 20th the FDA announced the release of its Final UDI rule. This marked an important step forward along what has been a long and rocky road towards supplies and medical devices having unique device identifiers. Unlike drugs which have long had a specific, unique NDC (National Drug Code) number which allows for identification and tracking, medical supplies/devices have had no such similar system - with each manufacturer assigning and using its own catalog number we have a situation where a given catalog number may be used by one manufacturer for a wound dressing, by another manufacturer for a urinary catheter, and by yet another for an implantable device...
It has long been recognized that the use of a standard naming convention and unique device identifiers would greatly benefit almost all the parties, from the supply chain (e.g. to simplify and facilitate inventory, recalls, etc.) to providers (e.g. for supporting the tracking devices implanted in patients, etc.) and eventually also patients (e.g. to look up information about the safety or effectiveness of devices).
The FDA Amendments Act of 2007 mandated that the FDA develop a system to implement a unique device identifier for medical devices. This led to a series of workshops, meeting, studies, etc. that in turn led to a proposed UDI rule being published in July 2012 and opened for comment. This eventually led to the issuing of the Final UDI rule in September 2013 (a little later than the mandated time frame of June 2013!)
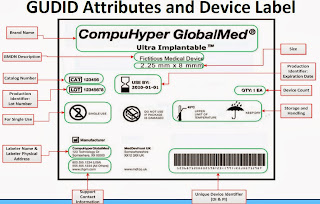
So, what is the UDI? In the words of the FDA:
"The Food and Drug Administration (FDA) has released a final rule requiring that most medical devices distributed in the United States carry a unique device identifier, or UDI. It also applies to certain combination products that contain devices and to devices licensed under the Public Health Service (PHS) Act (e.g., donor screening assays). A UDI system has the potential to improve the quality of information in medical device adverse event reports, which will help the FDA identify product problems more quickly, better target recalls and improve patient safety. In developing the proposed UDI system, the FDA worked closely with industry, the clinical community and patient and consumer groups, and conducted four pilot studies. A UDI is a unique numeric or alphanumeric code that consists of two parts:
- A device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device, and
- A production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
- the lot or batch number within which a device was manufactured;
- the serial number of a specific device;
- the expiration date of a specific device;
- the date a specific device was manufactured;
- the distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device."
A schedule with deadlines by which manufacturers have to obtain and label their devices with UDIs has been published, ranging from Class III devices which need to be labeled within a year to seven years when all Class I and unclassified devices need to be labeled with UDIs. There are many exceptions and special cases, for example Class III device makers can apply for a one year extension, Class I devices sold at retail do not require a UDI and may use their existing UPC code, etc.
The FDA's final rule allows for multiple issuing agencies - they will start off with three issuing agencies, GS1, Health Industry Business Communications Council, HIBCC, and ICCBBA, but will consider approving others that apply.
Finally, the benefits of the UDI as articulated by the FDA, include:
- Allow more accurate reporting, reviewing and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
- Reduce medical errors by enabling health care professionals and others to more rapidly and precisely identify a device and obtain important information concerning the characteristics of the device.
- Enhance analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources and registries. A more robust postmarket surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices.
- Provide a standardized identifier that will allow manufacturers, distributors and healthcare facilities to more effectively manage medical device recalls.
- Provide a foundation for a global, secure distribution chain, helping to address counterfeiting and diversion and prepare for medical emergencies.
- Lead to the development of a medical device identification system that is recognized around the world.
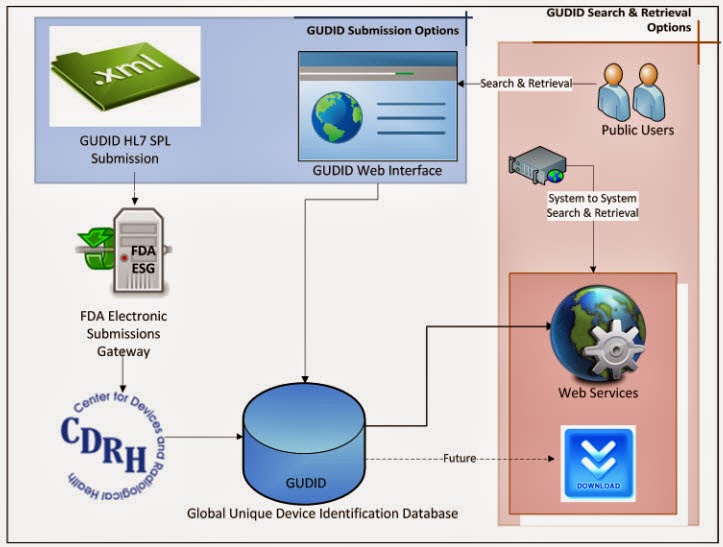
But enough about the UDI, this post is about the part that underpins the implementation, the Global UDI Database, GUDID or "Good ID" in FDA parlance... this database will house all assigned UDIs. The graphic below shows the possible interactions with the GUDID. Vendors will enter their devices and UDI information, either directly via web or electronically using a HL7 SPL transaction via the FDA Electronic Submissions Gateway. It is anticipated that the issuing agencies will develop electronic submission so that when vendors get a UDI (be it a GS1 GTIN or a HIBCC code, etc.) from the issuing agency the information will flow to the GUDID in an automated fashion. Providers, patients, etc. will either access the GUDID via web look-up or via Web services' search and retrieval.
Wisely the FDA is incorporating robust Master Data Management/Data Quality to ensure that the GUDID remains "clean.' The FDA has published a GUDID Draft Guidance, and will take comments through November 25th. So, perusing the draft guidance one has to ask 'Could the "Good ID" be better?'
GUDID "scorecard"
Click to enlarge:
Thinking forward to when all devices have UDIs and providers, patients, etc. are routinely using the GUDID, one would hope that the 'Good ID' would allow you to do certain things, including for example:
- The ability to see all packaging units of measure in which the base item is available e.g. EA, BX/5, CA/50 (each, box of 5, case of 500, etc.) - this appears to be supported by the GUDID.
- The ability to get all necessary item attributes from one source of truth. The GUDID is initially mandating a limited number of item attributes (see chart above), for example the presence of latex or natural rubber in a product, its MRI safety status, etc. There are so many other possible attributes that would be useful, it would be nice if the FDA could start off with a much larger initial set, understanding that going back and adding them later will overall end up to be more onerous than including them from the outset. Examples of other useful attributes could include the presence of DEHP, BPA, etc,: safety-related attributes (e.g. hazardous status, flammability, active or passive safety system if it includes 'sharps.' etc.); usage-associated attributes (e.g. the maximum number of permitted reuses for a 'reposable', etc.); environmental-related attributes (e.g. special disposal requirements, if recyclable, if green or containing recycled product, etc.); and so on...
- The ability to 'trace' predecessor or successor items. The GUDID requires that a device get a new UDI if the product is changed or if certain attributes change (e.g. if the item goes from containing latex to not, if an item goes from non-sterile to sterile, etc., since such changes essentially result in a new product). When a new UDI is obtained and the 'old' product is no longer manufactured, the old UDI is "retired" - the 'package Discontinue Date' is entered and the 'Package Status' field value is changed from 'In Commercial Distribution' to 'Not in Commercial Distribution.' However, if the item is still being manufactured in a new version with a new UDI it does not appear that you can pull up the discontinued item in the GUDID and see the successor (new) product UDI number... Conversely, you apparently cannot go back and see predecessor version UDI. With changes and improvements occurring at a fairly rapid pace for some devices this would be an important ability, as a provider could have items on its shelf past a 'Discontinue Date'. Also related to this is the fact that some device manufacturers have a habit of changing their catalog numbers... This blogger is not aware if a good (i.e. "real") business reason exists for such a practice, and like others has wondered if this is being done uniquely to impede the ability of providers or third parties to develop cross-references between 'equivalent' products made by different manufacturers! At the 2013 UDI Conference one manufacturer attendee asked if they were allowed to change the UDIs on items even if they were not modified or new (the answer was 'yes')! 'Tracer' functionality in the GUDID would go a long way to help in ensuring transparency.
- The ability to either see or link to the device's recall history. The GUDID is designed to only contain Device Identifier information and not Production Identifier information. Given that one of the major benefits of UDI implementation is to make recalls easier, one would hope that as vendors inform the FDA of recalls (which would include both DI and PI information) the corresponding historical recall PI information can be pulled up via the DI in the GUDID when researching an item. This would be invaluable in multiple instances, for example when a provider is choosing between a number of items (UDIs) to carry one input could be the items' recall histories; or if a patient is researching potential implants; etc. It is not clear that this will be possible with the GUDID as currently envisaged.
- The ability to look up the UDIs of all the items present in a custom pack or convenience kit (for when they are individually available commercially that is...). For convenience kits or custom packs where the constituent items are also in commercial distribution, it would be most beneficial to pull up a kit DI and see the DIs of the constituent items!
From a perusal of the draft guidance it appears that only one of these five is fully supported, while one is partially supported and three are not.
Additionally, also not clear to this blogger is the robustness of the GUDID. It needs to be robust, massively scalable, and always available. At the 2013 FDA UDI Conference mention was made of the GUDID being down for maintenance on the weekend. This may be OK in its initial phases, but once up and running with providers using it and 'pinging' it on a regular basis, this sounds problematic. A good model to follow could be one conceptually similar to that of the DNS system - if the DNS can support all the addressing needs of the web, with distributed servers and replication of records, and with the necessary scalability, it would be a good model (note: I'm sure the DNS is a lot more complex than this simplification, and I am no expert on the DNS, but am suggesting an analogous system be considered).
The bottom line? The answer to 'Could the "Good ID" be better?' is 'yes.' While it is ambitious and represents a breakthrough compared to current state, it could and should be even more ambitious and include these and other capabilities!
What do you think? Feel free to leave a comment...
UDI Resources:
FDA UDI page
GS1 and UDI
HIDA UDI page
Premier UDI page
AHRMM UDI page
AdvaMed UDI page
A simple guide to UDI
HIBCC UDI Resource page
The Healthcare Hub (GHX) UDI page
Brookings Roadmap for UDI Implementation
Note; All slides (except the "GUDID scorecard") courtesy of FDA, GS1, HIBCC handouts from the 2013 FDA UDI Conference.




No comments:
Post a Comment